Methods
Summary
The protocol for this project involves two domains: field collection and lab analysis.
Field Collection of Bald Eagle Nestling Blood Plasma Samples
Retrieving Eagle Nestlings and Taking Morphometric Measurements
Eagle nest productivity is quantified by aerial observation. Pilots fly low over nests and an observer records whether or not nestlings are present. Ground teams use flight report information collected during the aerial observations to narrow their field sampling to nests containing young in the correct age range (chicks ages 4-9 weeks). The field crew uses GPS coordinates taken by aerial observers to locate specific trees with active (containing young nestlings) eagle nests. Forest service certified climbers ascend the tree to the nest. Eagle nestlings ages four through nine weeks are safely removed from the nest and delivered to the crew on the ground (at this stage, age is estimated by developmental features of the bird). The ground personnel are trained in safely handling these birds without injuring themselves or the animal. Morphometric measurements of each chick are taken and used to (more accurately) estimate age and sex of each chick.
The following two pictures display the clear developmental differences evident in the eagle nestlings. The bird in the first photo hatched approximately 3 weeks later than the bird in the second picture. ( See Physical development of nestling bald eagles with emphasis on the timing of growth events)

Sex of the eagles is determined by measuring the length of their foot pad.
Collection of Blood Plasma Samples from Eagle Nestlings
Eagle nestling blood plasma is collected by permitted and certified individuals using aseptic techniques. The brachial vein is used for blood collection (this is the same vein used when humans give blood). The vein area is swabbed with an alcohol pad before injecting the needle. Approximately 10 milliliters (mL) of blood is collected from each eagle. Blood accounts for approximately 10% of the eagle's body weight and it is safe to remove up to 10% of their blood volume without injuring the bird. 10 mL is significantly less than 10% of the birds blood volume, and this process will not hurt the bird.
After the blood sample has been collected, a gauze pad is applied to the injection site to cause the blood to coagulate. The bird is then returned to the nest and the climber descends from the tree. The blood is centrifuged and the plasma is separated from the blood cell content. Samples are then frozen until they are analyzed in the lab.
Commonly Asked Questions
Do the adults attack the climber or the ground crew? No, they do not. The adults generally fly around the area, making loud calls but they do not attack. Eagles are a long-lived species and breed annually. Although they are unhappy about their chicks being removed from the nest, they do not risk injuring themselves.
Do the adults abandon the chicks because they have the 'human scent'? No, eagles do not have a very strong sense of smell. Some adults will return the nest before the climber gets to the bottom of the tree.
How do you catch the birds without them flying away? We sample nestlings who cannot fly. Eagles begin to fly around 11-12 weeks of age. The oldest chicks that we sample (9 weeks) are not able to fly.
Why do you use birds ages 4-9 Weeks of age? Eagles do not have fully developed thermoregulatory systems (i.e ability to control their body temperature) until they are 3 weeks old. Additionally, eagles begin to fly between 11 and 12 weeks of age but may try to prematurely jump. We use the 4-9 weeks age range to minimize potential harm to the chicks. In this age range they are old enough to regulate their body temperature but young enough that they are not likely to jump from the nest and hurt themselves.
Why bald eagles? Why nestlings? Our monitoring program is in the Great Lakes region, where eagles are year-around residents. They are top-level predators of an aquatic food web. Aquatic food webs are especially susceptible to accumulating environmental contaminants because of the tendency of contaminants to accumulate in fish tissues (they can either consume the contaminants directly in their diet or absorb them from the water column through their gills.) When eagles consume fish they accumulate contaminants contained in the fish tissue. Since eagles are the top of the food chain they experience the greatest levels of accumulated chemicals. Additionally, they are a large enough species to collect enough blood for analytical significance. We use nestlings because of their dependence on their parents for food. The average home range of adult eagles during the nesting season is approximately 3 miles. We can assume that contaminants accumulated in nestling plasma is from a local source.
How did you know where the nests were? Arial observers, who sample the entire geographic region, take approximate aerial coordinates of new nests. The ground crews use these approximate coordinates to locate the nests. New ground coordinates were taken for trees when they were located. These coordinates are recorded in a database to be used by future ground teams.
Lab Analysis of Contaminant Content in Blood
Lab analysis of the field samples allows us to quantify contaminants that have accumulated in the blood plasma of birds we sampled. These contaminants are removed from the plasma and then their concentrations are quantified using gas chromatography. After we quantify the contaminant content in each sample, we can compare these levels across the geographic region and through time.
Frozen blood samples are removed from storage and allowed to thaw. Plasma is treated with concentrated urea to denature the proteins in the plasma. This step increases effectiveness of the extraction procedures. Specialized extraction filters are used to remove (extract) chemical contaminants from the blood plasma. These filters must be 'conditioned' with methanol and water before they are ready to use. After the samples and extraction filters have been properly prepared and conditioned, samples are deposited into plastic cartridges containing the extraction filters. The plastic cartridge containing the extraction filter is pictured below.
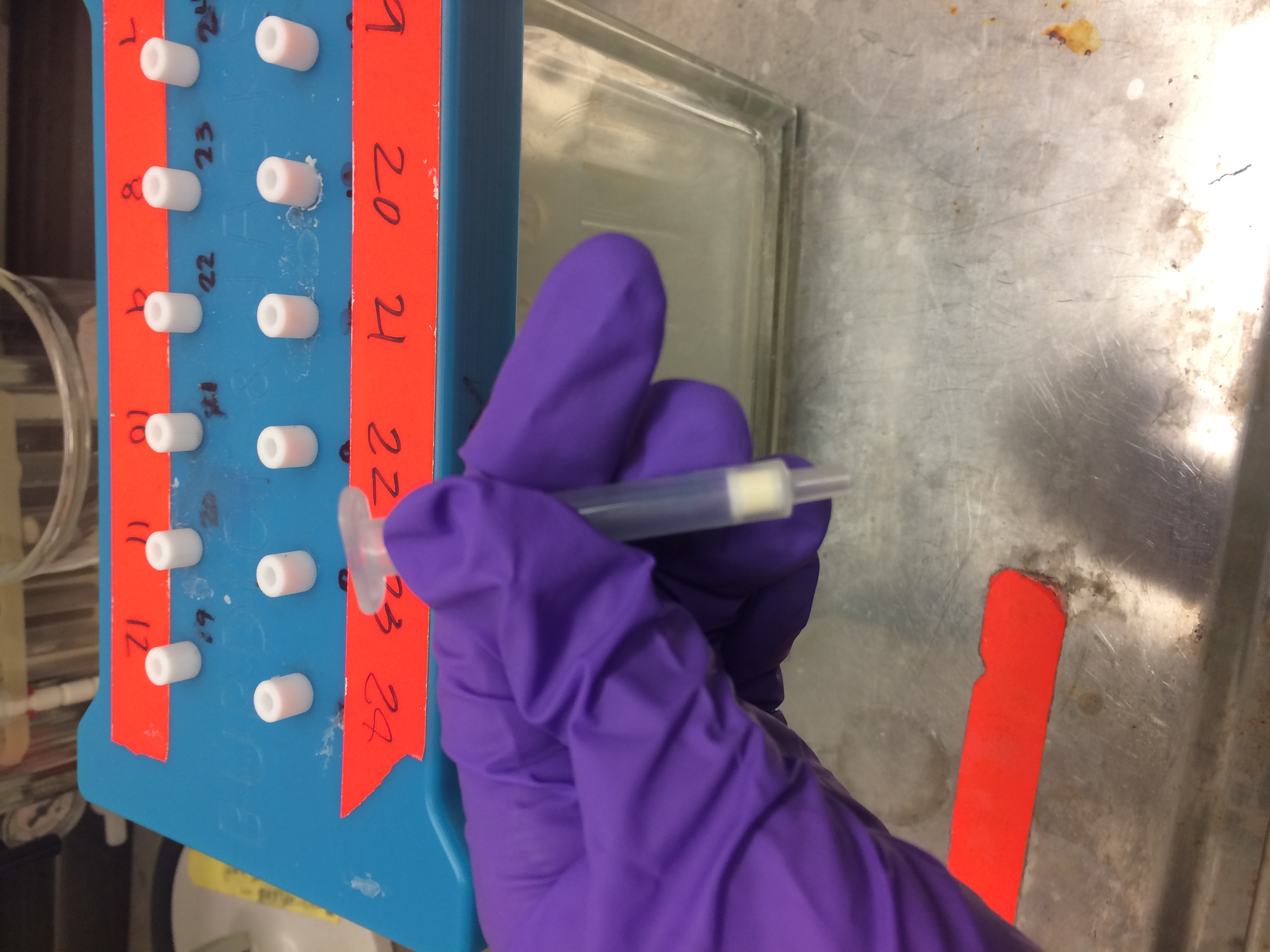
The contaminants must then be removed from the filter for analysis, a process referred to as "elution." New test tubes are put under the extraction filter to collect the filtered contaminants. Dichloromethane is deposited into the cartridge. This causes the contaminants to elute from the filter into the new test tube.
The contaminants are now suspended in solution and can be analyzed using gas chromatography. The solution containing the contaminants is put in specialized vials for gas chromatography analysis and put in the gas chromatograph (the machine that completes the chromatography analysis.)
Gas chromatography separates chemicals based on differences in their chemical properties. Liquid samples are heated up until they enter a gaseous state. The gas, known as the gaseous phase, then enters a long copper column lined with a liquid, known as the liquid phase. Each chemical reaches the gaseous state at a different temperature and interacts with the liquid phase in a unique way. Each chemical moves through the copper column at a specific time based on its specific properties. Therefore, each chemical will reach the end of the column at a specific time, known as a retention time. When contaminants reach the end of the column they are detected by an electron capture detector (ECD.) The detector records the concentration of chemicals at each specific retention time. We then know which contaminants are present in each blood sample and in what quantities.
Determining the Storage Capabilities of Extraction Filters
Normally, when completing an extraction, contaminants are eluted from the extraction filters immediately after all biological material has passed through the filter, and then analyzed in the gas chromatograph. We want to know how long contaminants can be stored in these extraction filters before being eluted for analysis, without losing analytical validity. Collection of contaminants in biological material is completed all over the world. Many permits are required to transport biological material across international borders. The Great Lakes region is bordered by the United States and Canada. We no longer collect samples from the Canadian side because of the complicated permitting process.
If contaminants can be stored inside these extraction filters, then transporting biological material is not necessary (after the sample passes through the cartridge no biological material remains in the cartridge-filter apparatus.) Contaminants could be extracted abroad and transported for domestic chemical analysis with little hassle. This would hasten the analysis process and allow for regulatory decisions with regards to the environment to be made sooner.
Each blood plasma sample will be deposited into 10 different extraction filters. One of these filters will be immediately analyzed for contaminant content. Each of the other 9 filters will be subjected to specific storage interval treatments. After the storage interval time has elapsed, the contaminants will be eluted from the extraction filters and analyzed for contaminant content. The contaminant recoveries for each sample from each treatment will be compared to determine if storage time has an impact on contaminant concentrations recovered from the filters.
Challenges
Chemical standards used for gas chromatography analysis are suspended in hexane which is highly volatile. Unfortunately, this means that the standards are only useable for approximately one month before needing to be replaced. Each time new standards are replaced they must be diluted to the proper concentrations and the gas chromatograph must be re-calibrated. This process can take up to a week to complete. Samples cannot be run until the re-calibration is completed, meaning that there will be a week gap in the time interval treatments. We will minimize the effects of this issue by working diligently on re-calibrating the gas chromatograph so that down time is minimized.
Pre Analysis Plan
If analyte retention in extraction filters is negatively related to storage interval length then as storage interval length increases analyte retention will decrease. I plan to take each of the contaminant recoveries within a repetition (originating from the same sample) and compare for statistically significant differences in contaminant concentrations. I will do this for each repetition. I will also analyze differences in contaminant concentrations between each time interval. For example, the difference in contaminant concentration from the immediately analyzed filter versus the shortest time interval, and then the shortest time interval versus the second and so on. This will help to identify the optimal storage time interval.
Protocols
This project has not yet shared any protocols.


