Protocol for the estimation of OSL ages
An OSL age is the ratio of the radiation dose in Grays (Gy) to the rate at which it was absorbed. Sophisticated instruments known as “luminescence readers” can reset excited minerals to the ground state by stimulating them with artificial light and recording their luminescence in response. Among natural minerals, quartz and feldspar are the most suitable geochronometers to date by OSL. In the case of feldspar, released luminescence is termed infrared stimulated luminescence (IRSL).
Luminescence intensity tails off with stimulation time and can be graphically represented by a decay curve:
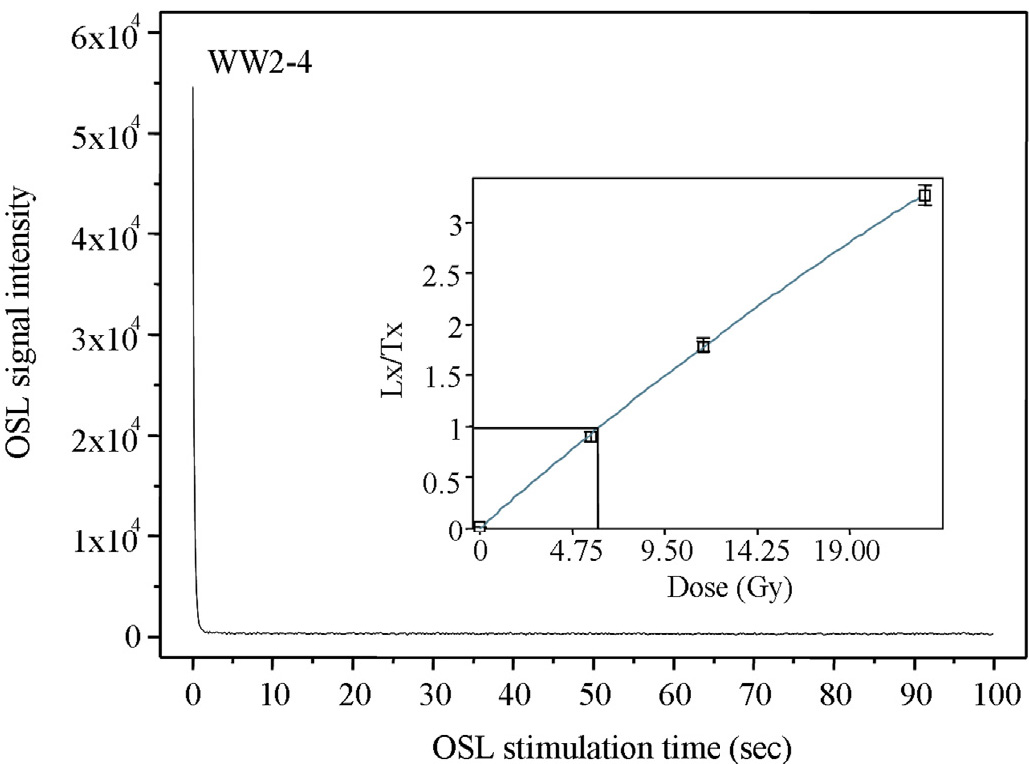
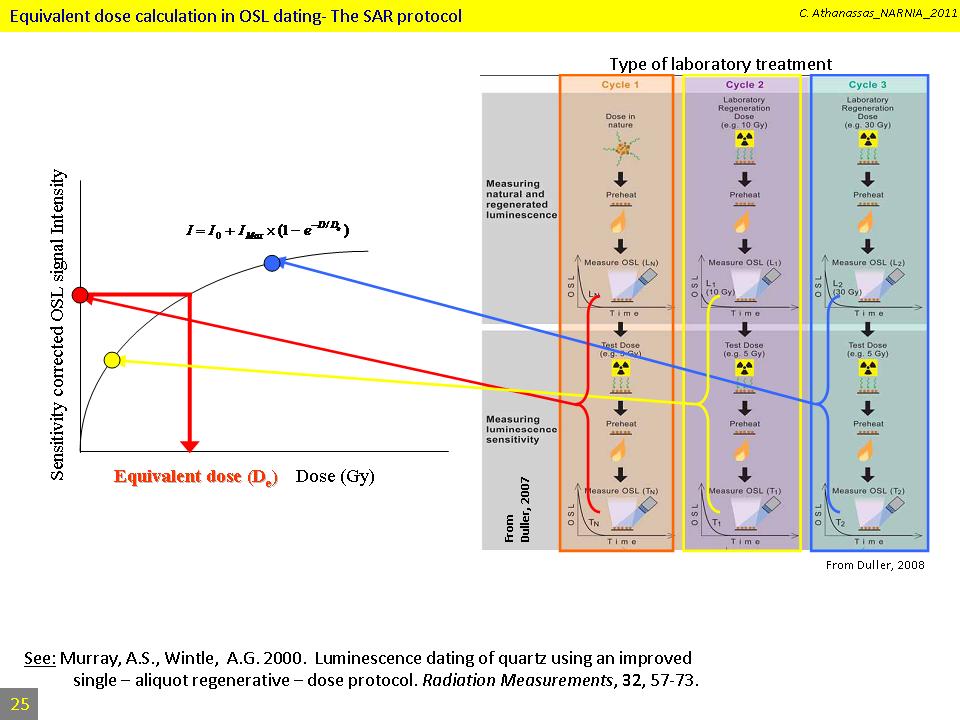
Luminescence intensity is more or less proportional to the dose. The laboratory equivalent of the natural dose, known as the equivalent dose (De). The De can be evaluated by interpolating the natural OSL signal intensity into a formula, either linear or exponential, that represents the best fit to an empirical plot containing pairs of known doses (regenerated doses) with their associated OSL signal intensities. The resulting plot is termed a “growth curve”. This method of equivalent dose estimation is known as the singe-aliquot regenerated (SAr) dose protocol (Murray and Wintle, 2000):
Below, the basic laboratory procedures involved in OSL dating and the individual steps of chemical and mechanical treatment for the purification of quartz are presented.
This flow chart summarizes all the field and laboratory steps to be taken for the evaluation of the equivalent dose, the dose rate, and finally the OSL age of a sample.
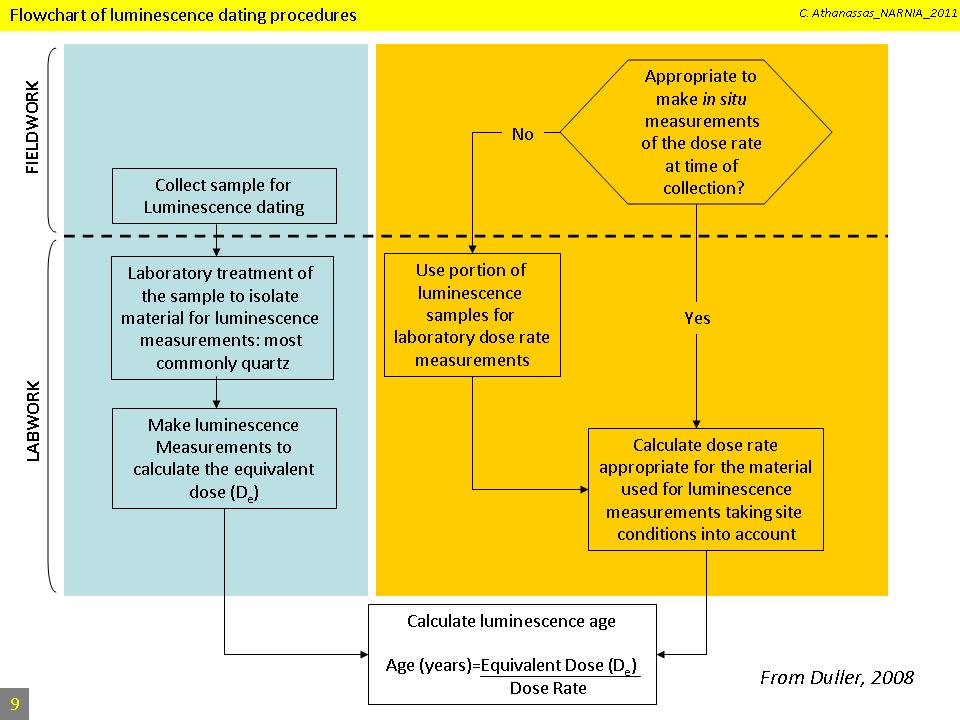
The procedure starts with fieldwork. Fieldwork involves sample collection, and in situ measurements of the radioactivity if this is considered as necessary. Samples are then brought into the laboratory and undergo several chemical and mechanical treatments in order to end up with the target mineral, which usually is quartz.
Specifically, samples are processed in dark rooms under red light which usually emit in the dark red part of the spectrum (at ~ 500 nm wavelength). This lighting conditions are considered safe as they allow scientists to work with comfort without affecting the stability of the light-sensitive OSL signal. Initially, the samples are treated with hydrochloric acid (HCl) in order to remove all carbonate minerals and cements, reducing the sample into loose sediment. This procedure is time-consuming, and depends on the degree of calcite cementation and carbonate content.
The next step involves the removal of any organic content. For this reason, each HCl-treated sample is bathed in hydrogen peroxide (H2O2) solutions for several days. The remaining clastic material is then mechanically sieved and optimum grain-size fractions are separated (e.g. 80-125 microns).
The sediment is then subjected for density separation in order to isolate quartz grains. The heavy liquid typically used is sodium polytungstate (SPT). The sieved sample is added to SPT solutions of 2.6 g/cm³. Feldspar is lighter than quartz and floats while quartz is denser and will sink at the bottom of the cylinder.
Afterwards, isolated quartz grains are attacked by hydrofluoric (HF) acid for a short time (45 minutes). This step aims to further purify quartz by dissolving any residual felspathic minerals which might have escaped the density separation stage. The HF-etched material is then briefly bathed in HCl to dissolve any byproducts of the HF-etching stage. Quartz is then split in sub-samples (aliquots) which are mounted on aluminium or stainless-steel disks sprayed by silicone oil. Typically, the aliquots used in the measurements are usually 0.5 cm in diameter.
Next, the aliquots are inserted into the luminescence reader in order to be measured. The equivalent dose of each aliquot will be measured by applying the SAR protocol discussed earlier. Special software allows communication between the user and the luminescence reader.
A more sophisticated means of treating statistically the apparent equivalent doses is plotting them as radial plots (Galbraith et al., 1999). They are useful in presenting graphically equivalent dose estimates that are adjunct by variable errors. Besides, one can exploit radial plots to identify different populations of equivalent doses making up a distribution (due to insufficient bleaching for example).
For homogeneous sedimentary layers or closely-packed and lithologically homogeneous mudbricks the estimation of the dose rate involves determining radioelement concentrations either by standard analytical methods, such as neutron activation analysis (NAA) or inductively coupled plasma mass spectrometry (ICPMS), or techniques based on the emission of α, β, and γ rays from the decaying nuclei, such as alpha- and beta-particle counting, or gamma spectroscopy. Radioelement concentrations are then converted to dose-rate units (Gy·ka−1) using conversion factors (Guérin et al. 2011) that deliver the dose rate per radioelement’s concentration (ppm).
- Published on Jul 19, 2016
- 63 views
- 0 comments
- Print this page
- Back to Methods