Experiment Protocol
METHODOLOGY
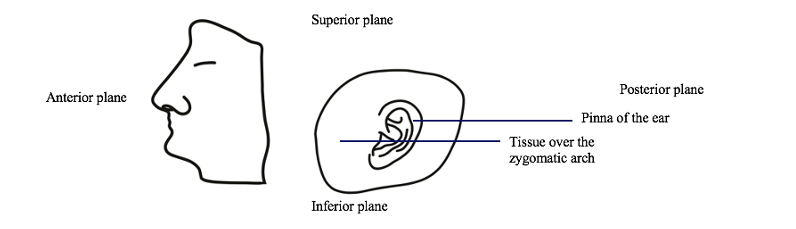
1. Specimen Preparation: Cadavers are being treated and embalmed according to protocol in the Advanced Technical Skills Simulation Laboratory (ATSSL). The cadavers will be lightly embalmed and will have been received by the ATSSL within the same week as our dissections will occur. There are two kinds of embalming that are conducted for pathological specimens: hard, and light embalming. Hard embalming is used to preserve the specimens long-term, and results in a specimen with low joint flexibility. Light embalming is used to preserve the specimens for a shorter period of time and leaves them with soft internal organs and highly flexible joints. Both embalming methods utilize formaldehyde, glutaraldehyde, methanol, humectants, wetting agents, and other solvents. The biggest difference in materials between the methods is the concentration of formaldehyde that is used. Hard embalming uses 37-40% formaldehyde, whereas soft embalming uses 5-35% formaldehyde. We have chosen to use light embalming because the flexibility makes our dissection process easier and because the lower concentration of formaldehyde will interact less with the internal structures, leaving them histologically more similar to how they were prior to embalming, resulting in more accurate analysis. Cadaveric specimens will be received by the study team as heads that have already been cut along the midsagittal plane. The area of tissue over the temporal bone and zygomatic arch including the pinna of the ear will be received as a separate piece. The skulls and brains will be removed from the specimens. All work will be conducted within the ATSSL and all dissections will be completed bilaterally. Refer to Figure 2 for an image of how specimens will be received.
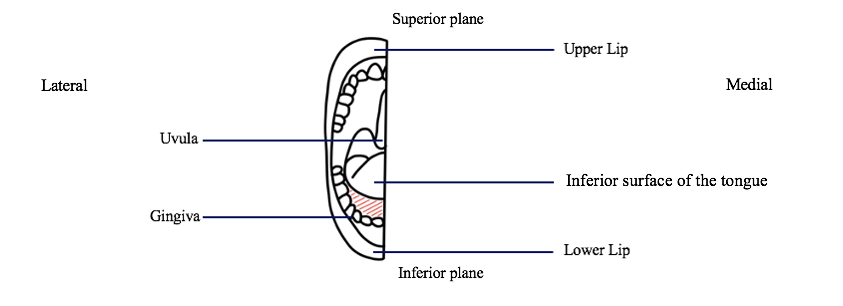
1.1 Sublingual gland dissection: Using scissors, the tissue along the gingiva and the inferior surface of the tongue will be spread apart and separated to reveal the sublingual gland underneath. The scissors will then be used to separate the margins of the gland from the surrounding muscle and fascia.
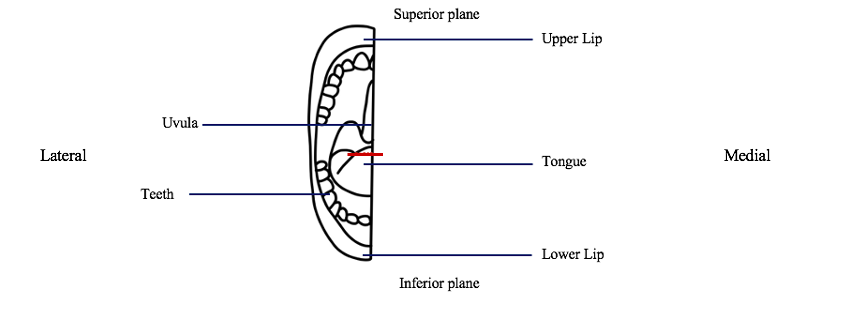
1.2 Lingual gland dissection: A 3cm x 3cm section of the tip of the tongue marked medially from the distal end of the frenulum to the lateral edge of the tongue will be excised using scissors.
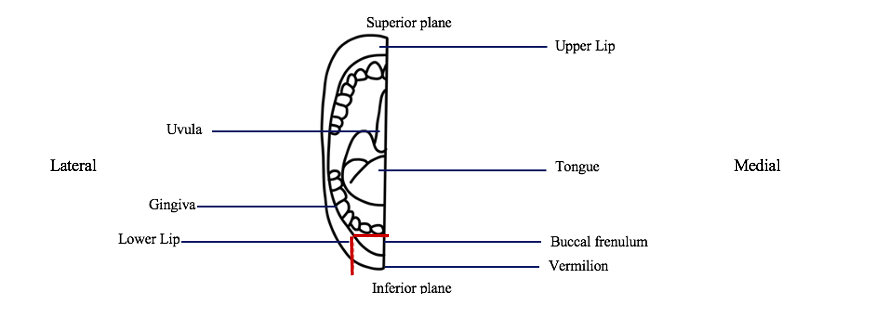
1.3 Labial gland dissection: A 3cm x 3cm section of the lower lip from the blade cut along the vermilion and buccal frenulum, along the gingiva and up the lateral edge of the lip will be excised using scissors.
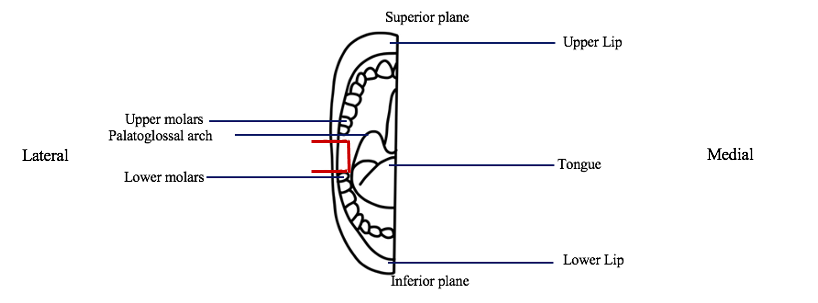
1.4 Buccal gland dissection: A 3cm x 3cm section of the cheek superior to the lower molars, inferior to the upper molars and lateral to the palatoglossal arch will be excised using scissors.
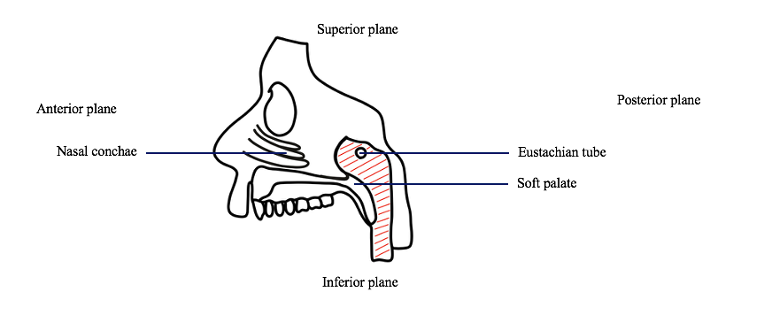
1.5 TG dissection: The tissue around the eustachian tube, superior to the soft palate and posterior to the nasal conchae will be excised using scissors.
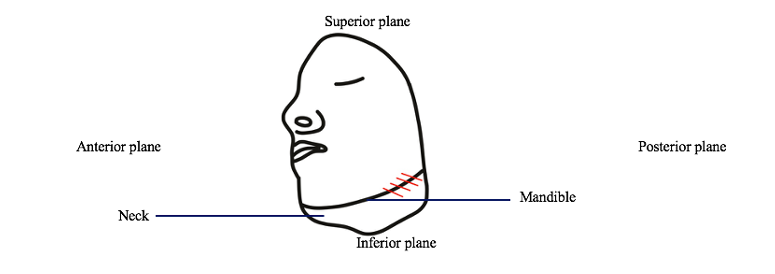
1.6 Submandibular gland dissection: Scissors will be used to spread and separate the soft tissue inferior to the mandible to locate the submandibular gland. Cuts will be made into the fascia encapsulating the submandibular gland until the margins of the gland are located.
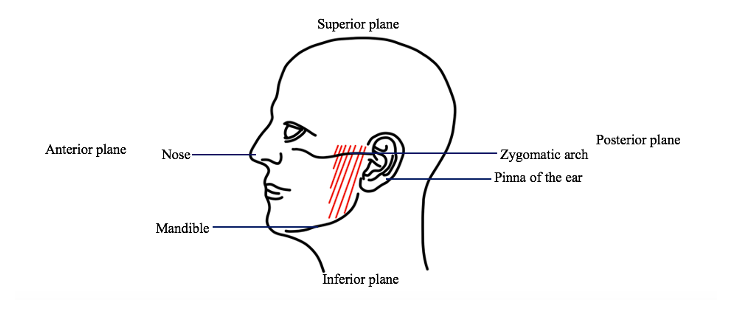
1.7 Parotid gland dissection: Scissors will be used to spread the and separate the soft tissue overlaying the temporal bone. Within these layers of muscle and tissue is the parotid gland. The scissors will be used to separate the margins of the gland.
1.8 Sample preservation: All samples will be laid out for photographing and will then be stored in 10 mL vials with 10% formalin for preservation. Samples will be stored at 8ºC. Each sample will be harvested bilaterally to maximize sample availability. The samples will be infiltrated with paraffin to allow for cell-support. Once the samples have been fully infiltrated and dried, 3 um sections of the samples will be prepared using a glass blade microtome, and then will be placed on glass slides. These samples will undergo both IHC with the PSMA-ligands and H&E staining. 2 IHC: We will be following a standard, established IHC protocol utilizing humanized PSMA-antibodies, which is outlined in Appendix 3. IHC is being used so that we can visualize the TGs’ PSMA-uptake levels in comparison to the SGs since PSMA uptake was a focal point of the Valstar et al. study, however, TG uptake of PSMA was not compared to the major and minor SGs’ uptake.
2 IHC: We will be following a standard, established IHC protocol utilizing humanized PSMA-antibodies. First, the samples will be stained with hematoxylin, which will stain the nuclei a purple colour. Once this is complete, a weak alkaline solution will be added to the samples, turning the nuclei a dark blue shade (18,19). Then, a weak acid alcohol will be added to the sample to remove any non-specific background staining. Next, the eosin counterstain will be added to the samples, which will stain the non-nuclear cellular components pink (18,19). Finally, a thin layer of polystyrene mountant will be applied to the samples, followed by a glass coverslip. This process will allow for differentiation between the cells of the glands as the nuclei will be stained a different colour from the rest of the cellular structures. IHC is being used so that we can visualize the TGs’ PSMA-uptake levels in comparison to the SGs since PSMA uptake was a focal point of the Valstar et al. study, however, TG uptake of PSMA was not compared to the major and minor SGs’ uptake.
3 H&E staining: We will be following a standard, established H&E staining protocol. First, the wax will be dissolved from the slides. Next, the samples will be incubated with various blocking agents and buffers, followed by an incubation period with the PSMA-ligands. After this is complete, the samples will be incubated with the human monoclonal PSMA-antibodies, followed by incubation with a secondary antibody for signal amplification. The last incubation will occur with diaminobenzidine solution. Staining intensity will be monitored with a light microscope and once the desired intensity is reached the sample will be rinsed with buffer. Lastly, the sample will be mounted using H&E staining. This process will allow for differentiation between the cells of the TGs and SGs as the nuclei will be stained a different colour from the rest of the cellular structures.
4 Tissue sample analysis: After the IHC and H&E staining processes are complete, comparisons between the TGs and major and minor SGs, as well as between male and female TGs will be made. Specifically, comparisons will be made regarding the serous and mucus acini density and volume, serous to mucus cells ratio, mucin index, and uptake of PSMA-ligands. Samples will be analyzed using a standard light microscope. Microscopy images will be analyzed using trainable Weka Segmentation machine learning software on ImageJ as well as area and density tools on ImageJ. Standard descriptive statistics and two-tailed unpaired t-tests will be used to analyze the results. A p-value of 0.05 will be considered significant.
- Published on Jan 21, 2022
- 0 views
- 0 comments
- Print this page
- Back to Methods