About This Project
Polyphenylenes are polymers with applications in batteries, hydrogen gas storage, and chromatography. Current methods of producing polyphenylenes are extremely expensive and produce a lot of hazardous waste. I will develop a method of making polyphenylenes using anisole-like compounds, Nickel (Ni) catalysts, and Magnesium (Mg) or Zinc (Zn) for about 0.5% of the cost. Besides the cost savings, the byproducts will be recyclable, and the method can be used in other syntheses.
Ask the Scientists
Join The DiscussionWhat is the context of this research?
Anisole-like compounds (anisoles) are cheap ($5.24/mol to $9.06/mol) and easy to functionalize, unlike the bromophenylboronic acids ($2578.65/mol, $3169.10/mol, $4004.55/mol) typically used to make polyphenylenes. While there is a method for coupling anisoles together, it requires an expensive diboron ($6663.76/mol). To make polyphenylenes affordably one needs to use cheap coupling agents like Mg ($2.37/mol) and Zn ($3.45/mol). While Mg and Zn have been used this way with arylhalides, it has not been shown to work with anisoles. Developing a method to couple anisoles using Mg or Zn, would also allow one to couple anisoles with esters, aldehydes, and several other functional groups - making this method a very useful synthetic tool.
What is the significance of this project?
This project has four significant points. First, the production of anisoles is safer and greener than making aryl halides and aryl boronic acids, and can be done using wood waste (lignin). Second, it develops the lowest cost method of coupling anisoles together, as well as a method for coupling them with esters, aldehydes, etc. making this method a versatile synthetic tool. Third, the reaction byproducts (methanol, Mg and Zn salts) are easily recycled, and can even reused in the overall process. Finally, it takes dendrimeric polyphenylenes from being a laboratory curiosity into a commercially viable product.
What are the goals of the project?
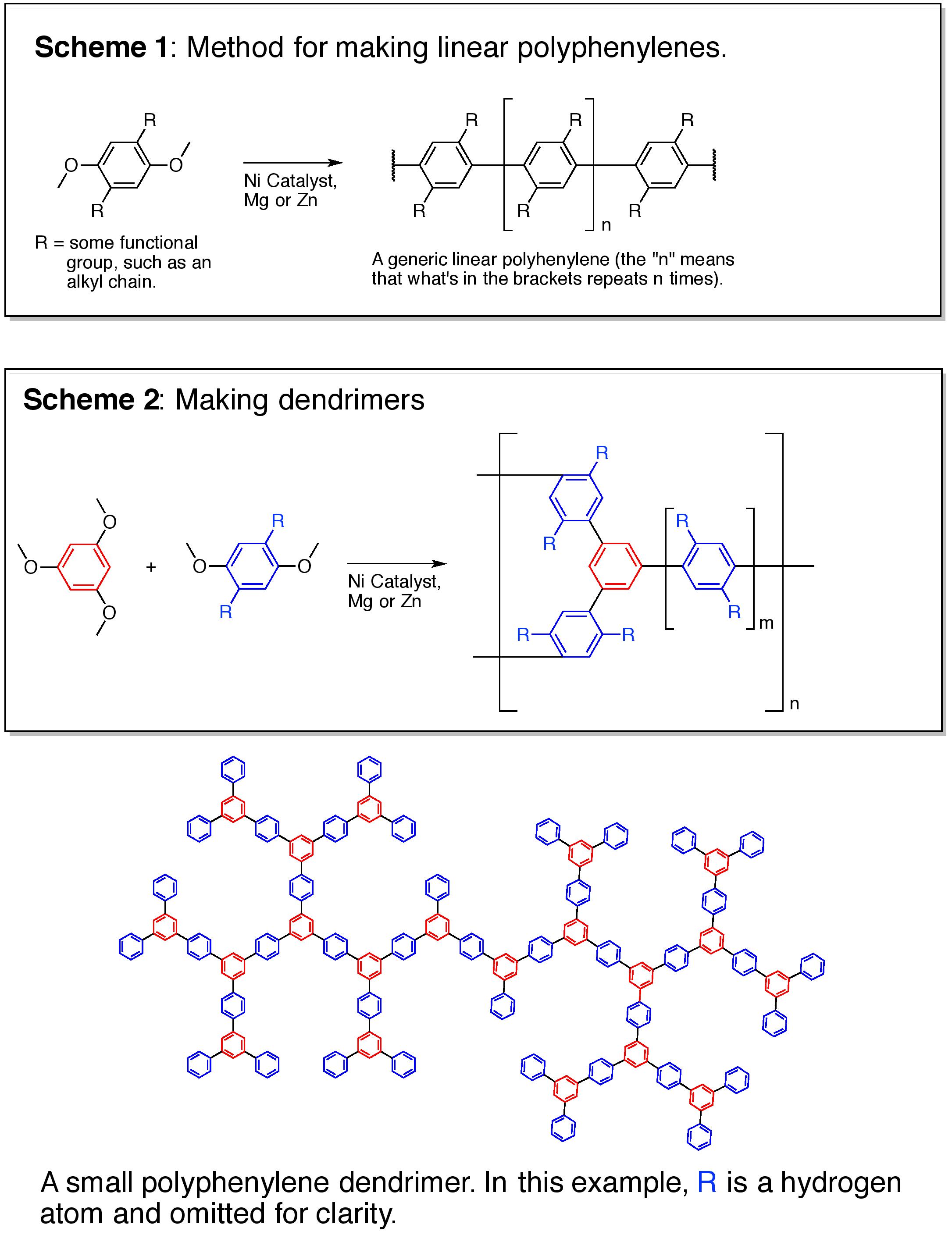
I will make several small proof-of-concept batches of polyphenylenes, varying both in branch length and functional groups off those branches (think leaves). The basic chemistry is shown in Schemes 1 & 2 with a small dendrimer shown below the schemes (see the additional information section). After the dendrimers are made, they will be tested for their ability to separate chemical mixtures (chromatography). The structures of all compounds made will be confirmed by NMR. This entire process will take about a month.
Budget
I'm currently at the proof-of-concept stage. Optimization and further development will come later. A successful proof-of-concept now, followed with a bit of optimization, will allow me to apply for a multi-year ~$250,000 National Science Foundation (NSF) grant in September 2016.
In order to confirm that I've synthesized my desired compounds, I need to rent NMR time.
Here are 9 of over 20 chemicals I will use:
1,4-DIMETHOXYBENZENE $68
1,3,5-TRIMETHOXYBENZENE $94
P-ANISIDINE $27
DI-TERT-BUTYL DICARBONATE $48
Anhydrous NICKEL(II) CHLORIDE $157
BIS(TRICYCLOHEXYLPHOSPHINE)NICKEL(II) $42
1,3-DICYCLOHEXYLIMIDAZOLIUM CHLORIDE $154
Anhydrous N,N-DIMETHYLFORMAMIDE $55
PHOSPHORUS (V) OXYCHLORIDE $41
Shipping and handling $230
Endorsed by
Meet the Team
Troy Wahl
I got my BA Mathematics and BS Chemistry from University of California at Santa Cruz, then taught high school math and science are 5 years. I then returned to the lab manufacturing peptides for Bachem. After that I continued working as an synthetic organic chemist doing industrial small molecule drug discovery and process development for 10 years. In 2009, I returned to school and earned his PhD Chemistry in 2013 from Portland State University. I'm currently struggling to get a cannabis consulting business off the ground (the industry wants his ideas but doesn't want to pay - he is not the only chemist facing this problem...) - life as a lab rat is so much easier and enjoyable (I enjoy chemistry the way most people enjoy sports).
Additional Information
I'm always looking for ways to do things better, and this project is no exception. From talking with people in the cannabis industry, it is clear that they need powerful chromatography tools to purify their extracts, but like many academic researchers most can't afford state-of-the-art chromatography equipment (UHPLCs run $100,000-$150,000, and industrial scale systems can run into the millions of dollars). So I started thinking about how to make high performance chromatography materials that were affordable, more stable than what is currently on the market, and could be used in low pressure systems.
Chromatography is a powerful tool for separating complex mixtures of compounds, and uses much less energy then distillation. The ideal chromatography material has the following properties:
1: Chemically inert - that way it will not react with the compounds one is trying to purify, and it won't degrade with use.
2: High surface area to volume ratio - the higher this ratio, the better the separation.
3: Very porous - more porous the material, the more easily solutions can pass through it (high flow rates, low pressure), which allows one to use less expensive equipment to purify compounds.
4: "Goldilocks" pore size - smaller the pore sizes results in better interaction between the material and the compounds being purified, but if the pores are too small the compounds can't enter the pores. Smaller pore sizes also requires higher pressures to force solutions through the material.
5: Rigid - if the material swells or shrinks upon changing solvents then the pore sizes change reducing the material's ability to separate compounds.
Dendrimeric polyphenylenes meet all of these requirements. Unlike silica, alumina, or zirconium based chromatography materials, polyphenylenes are completely inert to both acids and bases. Linear polyphenylenes are very rigid, with the smallest backbone diameter possible for any chromatographic material, allowing for the maximum surface area to volume ratio. Like the branches of trees, dendrimers have a lot of open spaces (pores). By controlling the length of branches (the linear polyphenylenes), one can tailor the pore sizes within the dendrimers. Since these dendrimers are rigid, they don't swell.
Project Backers
- 4Backers
- 10%Funded
- $204Total Donations
- $51.00Average Donation


