About This Project
Making new molecules that can emit light is important for improving the cost and performance of LED lighting. This project involves our recent discovery of a new method for making such materials. It is both technically simpler and creates less polluting byproducts than the current ways used to manufacture similar light-emitting compounds.Ask the Scientists
Join The DiscussionWhat is the context of this research?
Organic Light Emitting Diodes (OLED's) are arguably the future of lighting technology. However, their costs relative to traditional lighting solutions still remains high. One of the reasons for this is that the "organic" portion of the lights are composed of molecules that require many steps to make, adding cost and waste to the process. Thus, there is a significant need for the discovery of new and simple ways to make molecules that emit light. In order to do this, new bonds between two carbon atoms must be created in order to form new molecular architectures around a carbon-carbon linkage.
An additional important aspect of our work is the focus on so-called "green" chemical methods. Many traditional ways that these types of compounds are made involve throwing away significant quantities of toxic byproducts, including tin, bromine or chlorine. The discarding of these byproducts is necessary for those specific methods to work. Our method uses only oxygen (in the form of ketones) as the handle for forming our new carbon-carbon bonds.
Example for these types of reactions are shown in the "miscellaneous" section below.
What is the significance of this project?
This project aims to develop our new discovery on how to form carbon-carbon bonds. We have found that addition of a metal compound (such as chromium, molybdenum, or tungsten) to a ketone (a carbon-oxygen double bond) can result in the formation of a new carbon-carbon bond. The only byproduct of this process that we've identified so far is oxygen. This new method results in similar products as the "McMurry" coupling reaction. However, it differs in that the new method is significantly less difficult than the McMurry reaction (see the "miscellaneous" section for a picture of this reaction)
The long-term impact of this project is that although many products of theMcMurry reaction can be used as potential OLED materials, the difficulty and associated costs involved with traditional McMurry conditions prevents it's use in the OLED industry. We believe that our approach alleviates many of the problems that raise costs, and may allow these types of materials to be studied as useful LED's in the future.
One of the reasons that our method is easier is that we rely upon microwave heating to reach the temperatures necessary for the reaction (~150 degrees Celcius, or 300 degree Farenheit). This is where your help comes in - for each reaction we study we have to use a new reaction vial. Your donation will enable us to purchase additional cases of vials and significantly speed up our efforts in discovering what conditions work best to make the desired molecules.
What are the goals of the project?
The goal of our project is to discover the best conditions for making the desired molecules. In the short term, we will use this information to publish a research paper on the topic. We will also use the information learned in this project to guide us in developing this technology into one that is useful for chemists in an industrial setting.
In the long term, we want to utilize our approach to make molecules of interest for OLED uses. This will involve a long term collaborative effort at NC State and beyond, which will be started here with your help.
Budget
The money raised in this project will be used to purchase the reaction vials used in microwave reactions. These vials comes in cases of 100 (http://cem.com/discover-sp-vessels.html) and are single use for our reaction. We anticipate using ~100 vessels a month so your funds will keep us running through the end of the Summer.
For the stretch goal of $800 we will utilize the extra funds to help fund the purchase of a gas addition kit. This will allow us to study if addition of gases such as hydrogen can be used to improve the effectiveness of our method. http://cem.com/discover-sp-accessories.html
Endorsed by
Meet the Team
Team Bio
After graduating from Oceanview High School in Agat, Guam in 1996, Walter attended the University of Oregon. There, he received his B.Sc. in 2000 after studying he synthesis of phosphine stabilized gold nanoparticles in the lab of Prof. James E. Hutchison. From there, he went to MIT and received his Ph.D. in 2006 under the mentorship of Prof. Richard R. Schrock with a thesis that dealt with the single-site catalytic reduction of dinitrogen to form ammonia. He performed postdoctoral studies at Lawrence Berkeley National Laboratory with Dr. Heinz Frei, focusing on excited state electron transfer in silicate-based materials.He has been at NCSU in the Department of Chemistry since 2010 where his lab develops new materials for studying how light interacts with matter.
For more information, see http://www4.ncsu.edu/~wwweare/
Press and Media
Press coverage for my previous #Scifund Challenge Proposal "Artificial Photosynthesis at NC State"http://wunc.org/post/scientists-using-web-donatio...
http://www.technicianonline.com/news/article_da711...
Additional Information
Rewards! - At each tier you will receive, along with our thanks, the following.
$1 - We will send you an electronic copy of data produced using one of the microwave reactors along with an explanation of the experiment and the results. You will also receive an electronic copy of our first published work in this area when it comes out.
$10 - In addition to the first tier, we will send you a signed, physical copy of data produced using one of the reactors along with an explanation of the experiment.
$25 and up - Along with the previous tiers, you will receive acknowledgement in our first paper using your donation. We already did this for our first #Scifund Challenge (http://scifundchallenge.org/blog/2013/06/28/the-f...).
As soon as you make a pledge, we will contact you so you can receive your reward!

A "Stille" coupling - in this reaction the bromine (Br) and tin (SnBu3) are thrown away in order to link the two halves of the molecule.
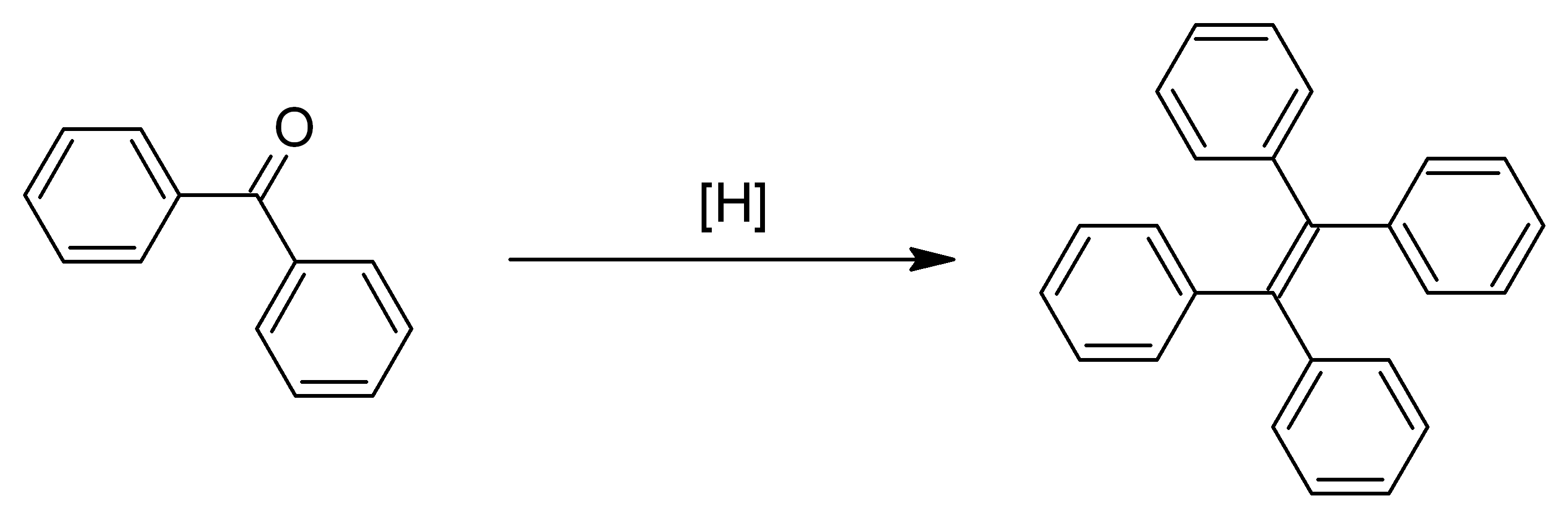
A "McMurry" Coupling - in this reaction the only part that's thrown away is oxygen (O) in the form of water (H2O). My project follows this approach.
Banner image courtesy of Mark Mosher on Flickr.
Project Backers
- 8Backers
- 268%Funded
- $1,075Total Donations
- $134.38Average Donation


