Methods
Summary
This study will follow what is known as a randomized, placebo-controlled trial. Participants have a 50/50 chance of being assigned to either the treatment (caffeine) group or a placebo. We are looking for approximately 30 men and women who are healthy but otherwise do not meet the existing physical activity guidelines of 150 minutes of moderate-intensity exercise per week (i.e. brisk walking). We are looking for 30 individuals as this will give us 15 individuals per group, which based on prior research, will likely be enough to detect differences between the groups (1)(2) over the 12 week period of the study.
Our recruitment strategy will be fairly simple - we will recruit using flyers posted on the college campus in addition to the campus-wide weekly e-mail newsletter. Additionally, we will run advertisements on Craigslist and recruit through word of mouth. All participants will need to complete a health history questionnaire assessing their overall health status and a physical activity questionnaire to determine their readiness to engage in exercise (3)
All groups will receive a FitBit activity tracker and given the same tests (a $150 value).

The caffeine group will take two doses of 3 milligrams caffeine per kilogram body mass (i.e.
180 lb individual = 82 kg = ~240 mg caffeine per dose = ~480 mg/day). They will be instructed to take one dose in the morning (0600-1000) and another in the afternoon (1400-1800). They will be given a 7 day supply each week, so that when they return for their new supply, data from their FitBit can be recorded . The same will occur in the placebo group except that they will receive an inert substance (i.e. corn starch or flour). A total dose of 6 mg/kg is relatively safe and well under doses that typically are associated with adverse side effects (≥ 9 mg/kg; LD50 for caffeine is ~150-200 mg/kg). Prior studies used 5 mg/kg so 6 mg/kg will help identify appropriate dosing for future research.
Body composition and resting energy expenditure will be assessed in the morning after a 10-12 hour fast and 14-hour period of alcohol, caffeine, and exercise abstention. They will be weighed in light clothing and waist and hip circumferences taken. For resting energy expenditure, participants will rest quietly in a supine position with a pillow and blanket. A ventilated hood will be placed over their head and they will remain in this position for 30 minutes, with data collected for a minimum of 15 minutes.
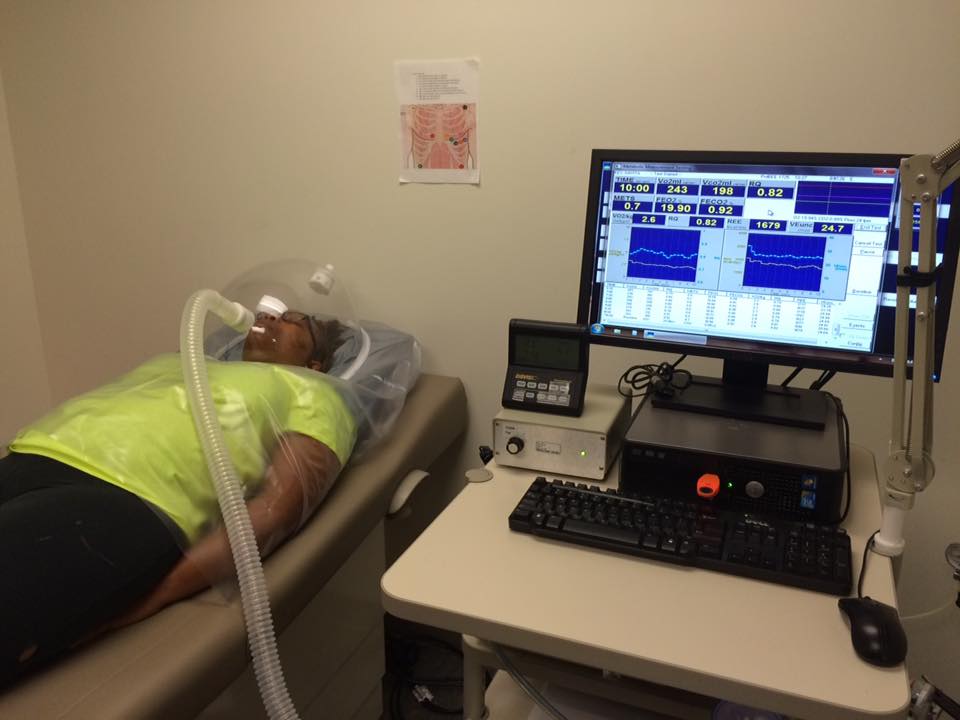
Following REE assessment, participants’ body composition will then be determined via air displacement plethysmography (BodPod). This system calculates body fat percentage, fat mass, and fat free mass based on displacement and resistance to air flow.
This visit should take about an hour. For 7 days at the start and end of the study, participants will also wear a combined heart-rate monitor/accelerometer (ActiHeart) to measure total energy expenditure; time spent in light, moderate, and vigorous physical activity; time spent sitting, standing, and lying down; steps per day; and sedentary time. The current gold standard for measuring energy expenditure is doubly-labelled water (DLW). DLW is a technique using water with isotopes of hydrogen and oxygen that are excreted in the urine. DLW requires collection of multiple urine samples, a sophisticated analysis plan, and is extremely expensive due to the scarcity of available isotopes. The best alternatives are so-called “multi-sensor” devices that combine accelerometry with one or more physiological variables. The ActiHeart combines accelerometry with heart rate monitoring to yield energy expenditure data that is comparable to that observed with DLW. Additionally, this device has minimal participant burden compared to DLW (no need to collect urine).

At the end of this 7-day period, participants will undergo a second BodPod test to allow us to calculate energy intake from changes in the energy balance equation, which is more accurate than self-reported food records. This requires some math and physics, but basically, but knowing the total energy expended and the changes in body composition, we can calculate the food eaten. Yeah science!
Challenges
The largest anticipated challenge will be participant retention. However, since they must visit us every week over the 12 week period, we will have ample opportunities to interact with them and discuss any issues they are having.
The other main challenge will be logistics as much of this study hinges on the purchase of equipment and the pending funding to help do so. However, we have multiple grants submitted internally and have hopes of at least one of them providing some funding to support this study in addition to your generous donations.
Pre Analysis Plan
We will analyze data using planned comparisons with statistical software (SPSS v. 23, Chicago, IL). Our primary aim is to determine whether caffeine increases physical activity and its domains (light, moderate, and vigorous activity). Our secondary aim is to examine if caffeine influences energy balance and it's components (total energy expenditure, resting energy expenditure, and energy intake).
Data will be examined to see if it is normally distributed (that is, the spread of our results (variance) follows normal statistical practice). We will utilize a repeated-measures analysis of variance to examine changes between groups at 0 and 12 weeks.
The primary findings will likely be presented in aggregate in a publication; however, opportunities will exist for multiple independent abstracts led by student authors at regional and national conferences such as Southeast American College of Sports Medicine and the national American College of Sports Medicine annual meetings.
Protocols
This project has not yet shared any protocols.