About This Project
US national reported that 1-2% of students are diagnosed with autism. Autism is difficult to treat because it is based on many subtle mutations in multiple genes. Interestingly, the Mexican cavefish shows traits that are very similar to this disease. The goal of this project is to investigate whether the cavefish's gene expressions are similar to human patients. We will then extract useful information which will facilitate the discovery of new treatment for autism.
Ask the Scientists
Join The DiscussionWhat is the context of this research?
Our goal is to promote the findings of new treatments in human autism spectrum disease (ASD) by investigating a new genetic model of the symptoms. ASD is diagnosed in 1-2% of human population, and is difficult to treat because many subtle mutations in genes cause complex reactions in brain development. Current mouse, rat and monkey models face a difficulty to address this multi-genetic point. A new model vertebrate, cavefish, Astyanax mexicanus, is composed of cave-dwelling and an ancestral-type, surface-dwelling populations. This cavefish may complement above mentioned model animals since cavefish shows behavioral and genetic similarities to ASD patients (Yoshizawa et al. soon to be submitted). We are addressing multi-genetic interactions of ASD genes in cavefish.
What is the significance of this project?
ASD research is a highly active-biomedical field. For example, neural cells induced from ASD patient cells (i.e. iPS cell) are used to investigate the defects at the cellular level, such as the synaptic function and cellular metabolism. Also, many mutant mice have been established whose ASDgene is manipulated into malfunctioning. These mutant mice inform the functions of each ASD gene. However, it is difficult to address the mechanisms of perturbation at the neural-circuit level, or at the gene-gene interaction level, with these methods. The cavefish model can fill these knowledge gaps, and has a strong advantage to highlight potential side-effects when the ASD genes are targeted by upcoming gene-targeting treatments (gene-therapy).
What are the goals of the project?
We have narrowed down major cavefish’s candidate genes to 15 by referring to their expression changes at the embryonic stage and their genomic locations, both of which are associated with behavioral/neurological changes (quantitative trait loci for behaviors). We will test whether or not these 15 cavefish genes show similar expressions to the genes in humans during later brain development through quantitative-PCR of cavefish brains.
Budget
Two of lab members, Nicolas Cetraro and Noah Simon have been trained for the cavefish work and the entire quantitative PCR (qPCR) process. Since we screen two pairs of qPCR primers in each gene, we need to run 30 primer pairs to check, and then 4 developmental points for each population (surface fish and cavefish). They will help with routine care of over 2000 fish in our facility during this project.
Others are consumables for qPCR will supply enough samples to finalize this project.
Endorsed by
 Project Timeline
Project Timeline
1st week will be for ordering materials and designing primers for qPCR, and 2nd-3rd week will be for test the primers to configure the best PCR primer sets, 4th-5th weeks for qPCR and 6th week for the data analysis
May 19, 2017
Project Launched
Jun 18, 2017
Ordering materials, designing primers and ordering
Jun 25, 2017
Configure the best primer sets and PCR parameters
Jun 29, 2017
Run qPCR for 15 genes
Jul 09, 2017
Analysis for qPCR results
Meet the Team
Team Bio
This team is focused on gene-expression, mainly performing molecular biology including quantitative-PCR (qPCR). This team is currently working on preliminary qPCR on ASD genes, and qPCR of extra-ocular light sensing gene (opsins) in cavefish.
Masato Yoshizawa
I have worked for more than 10 years on the cavefish genetics of behaviors by developing new assay devices, recording systems and bioinformatics scripts. I was also trained in the fields of mouse neurobiology, cell and molecular biology and developmental biology during my Ph.D. These facilitated me to conduct researches in integrated biological fields. Research from my laboratory aims to reveal the evolutionary and neural mechanisms that lead to the expression of adaptive behaviors in a given environment. Beyond this ASD project, cavefish gene function will be reversed back to the level of surface fish by inhibiting or overexpressing genes of interest (with CRISPR-on/-off, or Transgene). This could reveal how neural wirings are modified, and how evolution avoids side-effects of genetic changes. This project is, therefore, the core of what our laboratory focuses on.
Additional Information
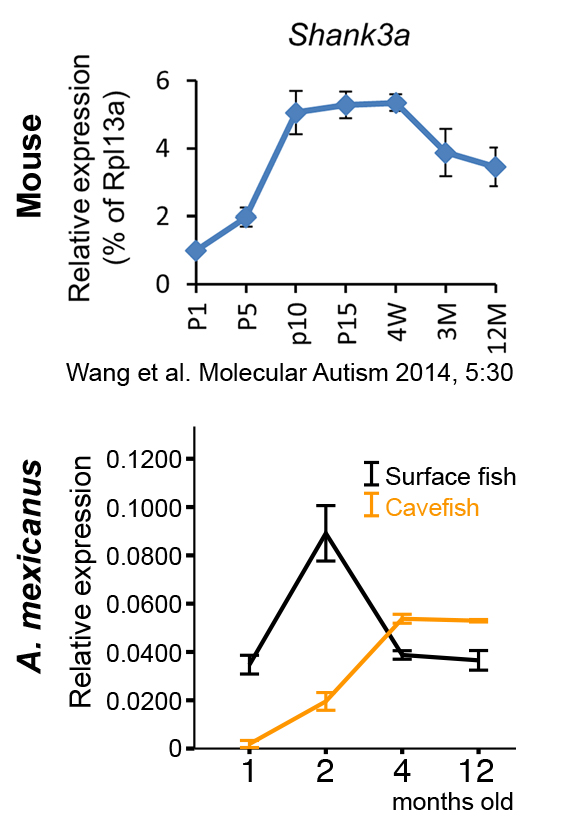 How similar are cavefish to ASD patient? Of course, human and fish are two very different species, but shares many aspects as vertebrates. By comparing with surface fish, ASD-like symptoms are highlighted in the cavefish: hyperactivity, asocial tendency, more repetitive behavior, higher cortisol (anxiety hormone) level, difficulty in sleep, and adhesion to a particular stimulus/object (see VIDEO "Vibration Attraction Behavior" https://www.eurekalert.org/pub...).
How similar are cavefish to ASD patient? Of course, human and fish are two very different species, but shares many aspects as vertebrates. By comparing with surface fish, ASD-like symptoms are highlighted in the cavefish: hyperactivity, asocial tendency, more repetitive behavior, higher cortisol (anxiety hormone) level, difficulty in sleep, and adhesion to a particular stimulus/object (see VIDEO "Vibration Attraction Behavior" https://www.eurekalert.org/pub...).
Additionally, when we looked at the expressions of the genes, which are homologs of human ASD risk gene, > 70% genes (295 out of 419 genes) have significantly changed their expressions in larvae (infant) stages. Some of the preliminary data started revealing the similarity of gene expression timing. The attached figure shows the one of the famous ASD gene, Shank3, which show the bump of the gene expression in post-natal of mouse and human (showing mouse case). This bump is correlated with the shift of splice variant from the embryonic form to the juvenile-adult form of Shank3. ASD patients cannot shift the splice variant, so the Shank3 gene keeps having the embryonic form. Surface form of A. mexicanus showed the bump, similar to a healthy individual, but the cave form did not have the rise, indicating the similarity with ASD patient.
Project Backers
- 5Backers
- 5%Funded
- $354Total Donations
- $70.80Average Donation


