Methods
Summary
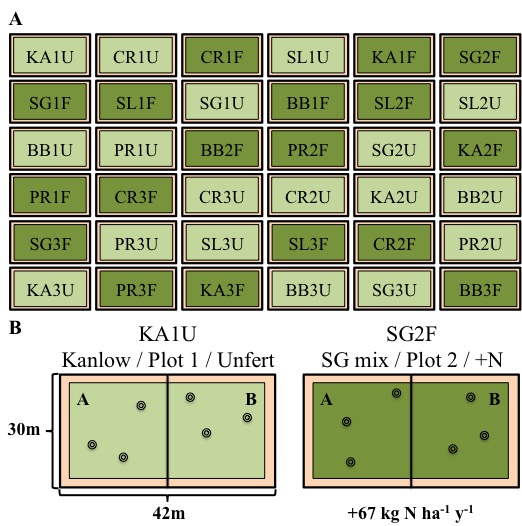
The Argonne National Laboratory located in Batavia, IL has an environmental research park that hosts a sustainable bioenergy feedstock production experiment, which was established in 2007. The goal of the bioenergy feedstock experiment is to determine how soil microbes can help increase plant biomass and biofuel quality, while minimizing expensive and environmentally detrimental fertilizer applications. Below is a schematic of the experimental site, and sampling locations for my "Sun to Soil" experiment. Briefly, I will perform three replicate 13-C labelling and soil samples in each plot containing monocultures of Panicum virgatum (switchgrass) that are fertilized or unfertilized.
Some background information...
Mycorrhizal fungi, in this case arbuscular mycrorrhizal (AM) fungi, form obligate biotrophic relationships with plant roots, where the plants give photosynthetically-derived carbon to the fungi for water and nutrients (nitrogen or phosphorus) that have been scavenged from small (hard to reach) soil pores and aggregates. See the figures below for some graphical representation of the plant-AM symbiosis in action.
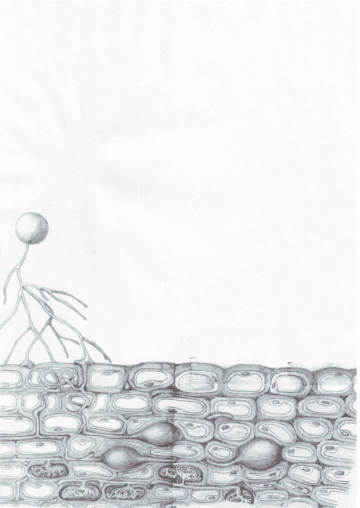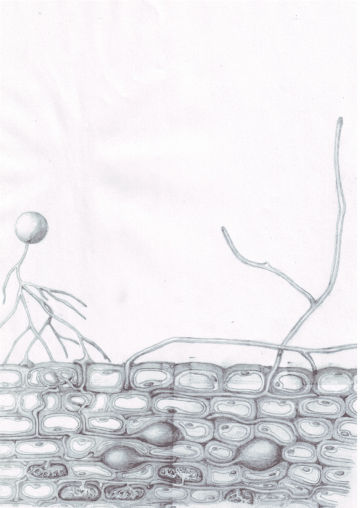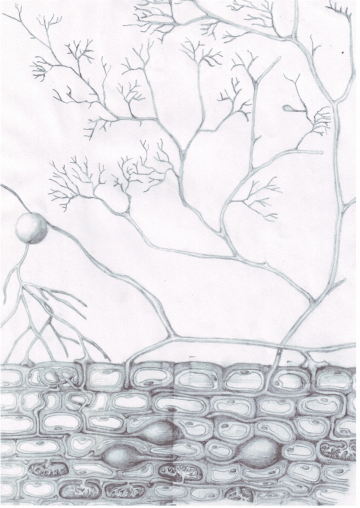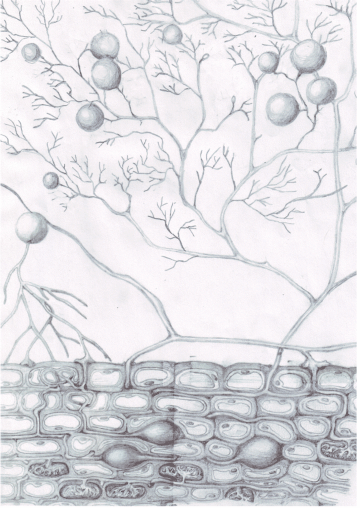
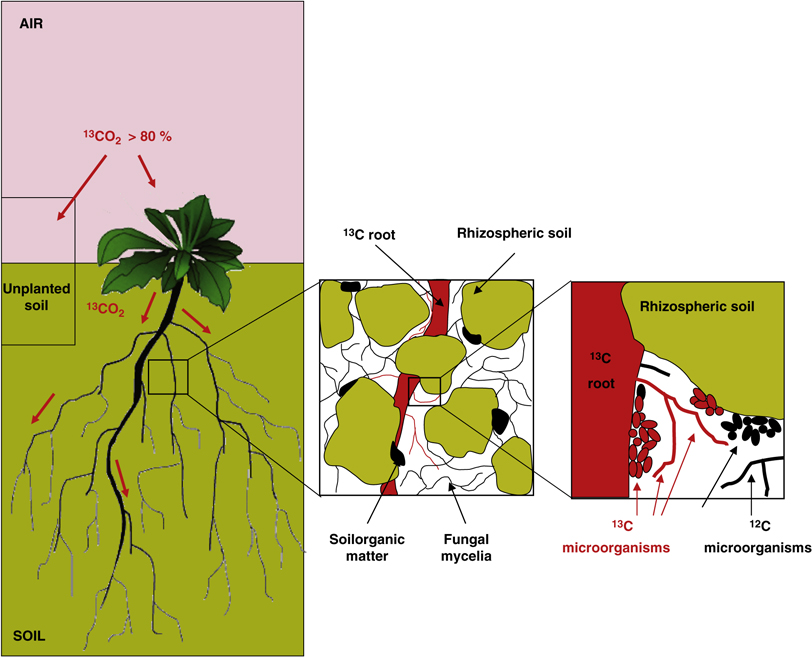
The figure below, from Bressan et al (2013), depicts the basic experimental premise of this project, where 13-C labelled CO2 is taken up by the plant aboveground, transferred to roots and then soil microbes, including mycorrhizal fungi and bacteria. I will be analyzing the mycorrhizal fungal communities that take up the 13-C, because it will be a direct, rather than indirect transfer of labelled C at the root-mycorrhizal interface (see the next figure).
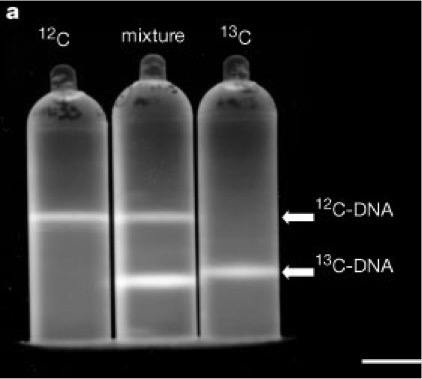
Quantitative stable isotope probing is a technique to identify the microbial communities that have taken up a 'labelled' isotope, in this case, carbon. What is this labelling I keep talking about? Under normal circumstances in the environment carbon is in the isotope form known as 12-C, but if I apply the heavier isotope of carbon, 13-C, I can physically separate the 12-C from the 13-C. These heavier (or labelled) carbon isotopes are processed like the normal 12-C, but when incorporated into the DNA of plants and subsequently soil organisms I can extract just the 13-C DNA (see the picture below).
Protocols
This project has not yet shared any protocols.