Methods
Summary
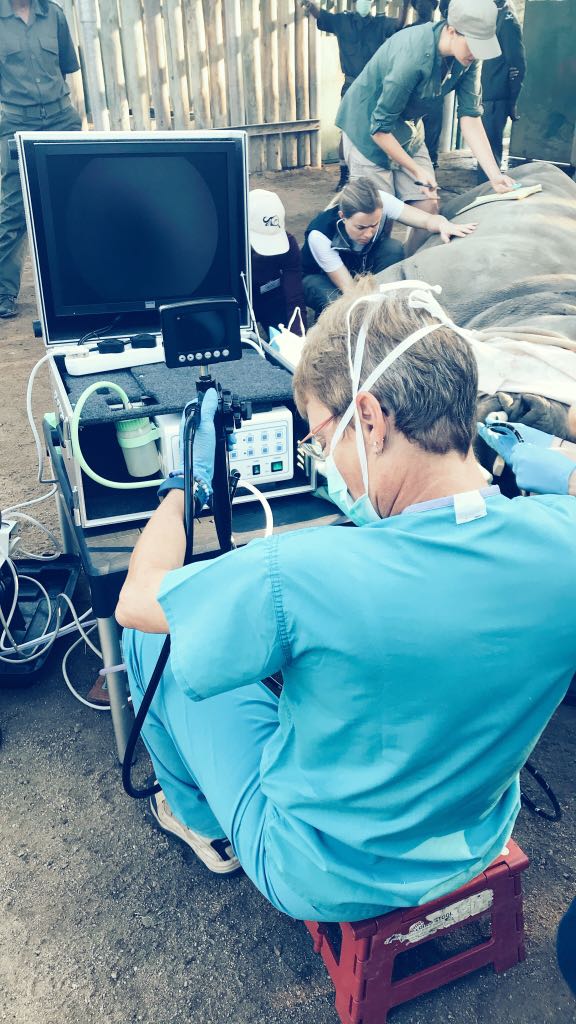
We will be using banked and freshly obtained samples from known infected and uninfected black and white African rhinoceros (Diceros bicornis, Ceratotherium simum) in order to develop our blood and direct detection (DNA) polymerase chain reaction (PCR) test. Sample types to be used for direct detection are respiratory samples (bronchial alveolar lavage) and blood (Lithium heparin stabilized) samples for the measurement of released antigen-specific immunological blood biomarkers. Bronchial alveolar lavage (BAL) samples will be collected by instilling saline into the alveoli of the lungs by the use of a specially adapted horse endoscope (see attached photo) and recovering it after a short incubation time. Blood will be collected from a vein in the animal's ear. Samples have already been collected from over 100 rhinoceros from M. bovis endemic areas in South Africa (SA). Additional samples from a minimum of 50 animals will be collected during the study. Blood immune biomarkers (IFN-y and IP-10) secreted after the incubation of the blood with tuberculosis-specific antigens will be measured by the use of an commercially available Enzyme-Linked Immunosorbent Assay (ELISA) previously identified to work optimally for this species and after some proper optimizations. Cut-off values will be calculated using culture-confirmed infected and uninfected rhinoceros, and relative sensitivity and specificity will be determined. The ELISA will then be used to assess presence and prevalence of TB infection in free-ranging rhinoceros in SA. Ancillary to this, the commercially available human TB DNA test called the GeneXpert MTB/RIF Ultra assay will also be used for the direct detection of TB DNA in the respiratory BAL samples as a way of identifying potentially infected animals under 2 hours or less with the hope of knowing which animals we should focus our attention on.
Challenges
The largest challenge for these type of wildlife projects is the safety of the animals and that of the scientists collecting the samples. Another important challenge is collecting enough samples from enough free-ranging animals. The Animal TB Group together with the South African National Parks have previously been faced with these challenges for many years and have developed numerous standard operating procedures specifically to avoid these and also to overcome them if they happen. Safety: During sample collection from rhinoceros either in the bomas or in the field there are always plenty highly qualified wildlife veterinarians present to avoid injury or death of the animals. If you look at the photo posted in this section you can see two veterinarians in the background monitoring the vitals (heart rate, breathing and CO2 emission) of the immobilized animal to avoid injury or death. In the background you can also see the heavy presence of game rangers specifically trained to handle sedated animals and to keep everyone safe during sampling of such animals. Samples: BAL and blood are collected by veterinarians that fully understands the importance of biological replicates and volume when it comes to science. This definitely overcomes our sample quantity challenge. With field sampling the mayor challenge is booking and affording the time of a helicopter and pilot. In parks rife with poaching these two commodities are like hen's teeth, however the Kruger National Park have always in-kind provided us with both resources when we needed it. We have overcome this by understanding that their pilot's are always on-call and that sampling animals may sometimes only happen ad-hoc. Again, this challenge have highlighted the importance of optimally sampling endangered wildlife when they are immobilized. You do not know when you will get another chance.
Pre Analysis Plan
We first plan on identifying an ELISA able to identify native rhinoceros biomarkers such as IFN gamma, IP-10 and more. Then we will identify the biomarker/s able to distinguish between the infected and uninfected (culture confirmed) cohorts by statistically comparing their measured concentrations between the two groups. Cut-off values will be calculated using Receiver Operating Curve (ROC) analysis and Youden's index. The relative sensitivity and specificity will then be determined for the identified optimal cytokine ELISA assay. This test will then be used to assess the presence and prevalence of TB infection in free-ranging rhinoceros. For the human TB DNA test, the GeneXpert MTB/RIF Ultra assay, BAL samples spiked with known concentrations of bacteria will be tested in order to determine the feasibility of using this test in these sample matrices and species. A presumptive limit of detection will be calculated in this species and samples obtained from free-ranging rhinoceros will be opportunistically tested for the presence of TB DNA and compared to the same sample's culture result. All cultures will be speciated using an established region of difference PCR (Warren et al., 2006).
Protocols
This project has not yet shared any protocols.