About This Project
Insulin is a hormone which supports growth of normal cells, but also of cancers. A very low carbohydrate (VLC) diet safely and readily lowers blood insulin levels. The effects of a VLC diet has potential to inhibit cancer growth without harming normal tissues.
We further hypothesize that a VLC diet can combine with the effects of drug treatment in cancers, thus reducing drug doses and therefore toxicities. We aim to test this hypothesis in cell cultures.
Ask the Scientists
Join The DiscussionWhat is the context of this research?
Very low carbohydrate (VLC) diets reduce insulin secretion to a minimum and also increase blood concentrations of ketone bodies. Ketone bodies were shown to inhibit cancers in mice and cells 30 years ago, but little follow up was done until recently. We've shown in preliminary cell studies that ketone bodies inhibited growth of human cancers, leaving normal cells unaffected. We also demonstrated that a very low carbohydrate diet was safe in patients, and that higher ketone body levels correlated with better outcomes. High blood ketone bodies may be very important as a cancer adjunct to drug treatment.
Our preliminary work tested effects on cancers due to insulin inhibition alone. We now aim to test VLC diet effects together with drug therapies, something that has not yet been done.
What is the significance of this project?
A VLC diet is particularly promising in combination with drugs. This approach can improve the overall efficacy of the treatment while simultaneously reducing the toxicity. We propose new laboratory studies to provide proof.
The recommended diet in the U.S. is a 'low fat' diet, less than 35% fat for adults. This diet provides far too many carbohydrates (40-60% of calories), 90% of which are sugars or starches, all stimulating excessive insulin secretion, resulting in type 2 diabetes and obesity. Obesity and type 2 diabetes are highly correlated with the development of a wide variety of cancers.
We have already obtained lab and human evidence of the feasibility of a VLC diet as a means to inhibit cancer growth.
What are the goals of the project?
To date, our cell culture experiments have shown that cancers produce less ATP (the energy molecule) and grow more slowly when ketone bodies (KBs) are added to ordinary growth medium. The added KBs cause inefficient ATP production because of a protein found in excess only in the cancers and not in normal cells.
Our immediate goal is to add a chemical to reduce this "inefficiency" protein. We predict that this chemical will increase ATP production in the cancers increasing ther growth. This would help prove our proposed mechanism. We also must test treatment drugs together with ketone bodies to see whether toxic drug doses can be reduced while maintaining or improving efficacy.
We wish to define clearly how VLC diets and ketone bodies can be used to treat cancers better and more safely.
Budget
The 'war against cancer' has resulted in very expensive and toxic drug therapies, and even more expensive personalized genetic approaches to treatment. On the other hand, our dietary approach has been shown to cause metabolic changes that inhibit a wide variety of cancers at low expense and reduced toxicities. The lab data we must still obtain is critical toward advancing toward human studies. For this, we need cell culturing materials, reagents and support for the laboratory to test our hypothesis.
Stretch Goal:
We will test all ten of our cell lines under all prior conditions, and for directly measurable enzymes (e.g. lactate dehydrogenase, pyruvate dehydrogenase) and glycolytic and respiratory substrates (such as lactate, citrate, oxaloacetate, pyruvate). This is called 'metabolomics' and is a significant addition to what we've been doing for the past 7 years. This is more expensive specialized testing as we must ship aliquots of our cells (frozen in dry ice) to a private company.
Endorsed by
Meet the Team
Affiliates
Eugene J Fine
I'm a nuclear medicine physician at Albert Einstein College of Medicine in NYC. Before medicine I obtained an M.S. in nuclear physics from U. of Pennsylvania. My work in nuclear medicine has often required PET (positron emission tomography) scans in patients with cancer, using 18F-fluorodeoxyglucose (FDG), a radiotracer taken up in cells which use glucose (including cancers).
I was fascinated with the "Warburg effect" in medical school-- ie that many cancers require anaerobic glucose metabolism--glycolysis--even in a normal oxygen environment. This glycolysis dependence is the basis of PET FDG scanning in cancers. It also provides a clue to understand why carbohydrate restriction can metabolically inhibit cancer growth, (though not simply because glucose in the blood is reduced; rather because low insulin and high ketone bodies interfere with glucose metabolism in cancer cells!!) In 2003 I started examining ketogenic diets. These diets, then largely ignored, were actually quite helpful for many people with obesity, diabetes and lipid disorders. It occurred to me that ketosis, which represents maximum insulin inhibition, could also inhibit cancer growth. Mice and cell culture studies from 30 years ago had suggested this. Reading more papers then helped me arrive at a consistent and highly plausible biochemical hypothesis.
I've worked with my colleague Richard Feinman since that time and we've now accumulated cell culture data which describe a distinct metabolic mechanism where ketone bodies inhibit cancer growth, leaving normal tissues unaffected. We showed preliminary proof of this hypothesis in 7 cancer cell lines as well as in a pilot ketogenic diet study in 10 patients with aggressive cancers. In cells (and people) ketosis was well tolerated by normal tissues and correlated with better outcomes.
I find this line of research quite exciting as it suggests less toxic, yet more effective future cancer therapies.
Richard David Feinman
Richard Feinman is Professor of Cell Biology at State University of New York Downstate Health Sciences University. His current research projects address the effect of ketone bodies on cancer cells in culture and the effects of ketogenic diets. These studies represent a confluence of his interests in energy metabolism, stimulus-response coupling in cells and the effects of diet, particularly those based on carbohydrate restriction and the ketogenic state. Dr. Feinman is principal author of the 26-author comprehensive review on "Dietary carbohydrate restriction as the first approach in diabetes management."
His recent book “Nutrition in Crisis” summarizes his world view on the interactions of nutrition and disease and he has pointed out that "If it turns out that we learn to treat diabetes by learning to treat cancer, it would not be the strangest thing that ever happened in science."
Dr. Feinman’s work is stimulated by, and continues to influence, his teaching in the Medical School where he has been a pioneer in incorporating nutrition into the biochemistry curriculum. Dr. Feinman is the founder and former co-Editor-In-Chief (2004-2009) of the journal, Nutrition&Metabolism. Dr. Feinman received his BA from the University of Rochester and he holds a PhD in chemistry from the University of Oregon.
Press and Media
The National Cancer Institute described our research in its Bulletin (click link and scroll toward the bottom to see a blue Box)
National Cancer Institute Bulletin
View a presentation video describing the hypothesis in some detail, delivered at the Ancestral Health Symposium in 2012: Carbohydrate Restriction and Cancer
Additional Information
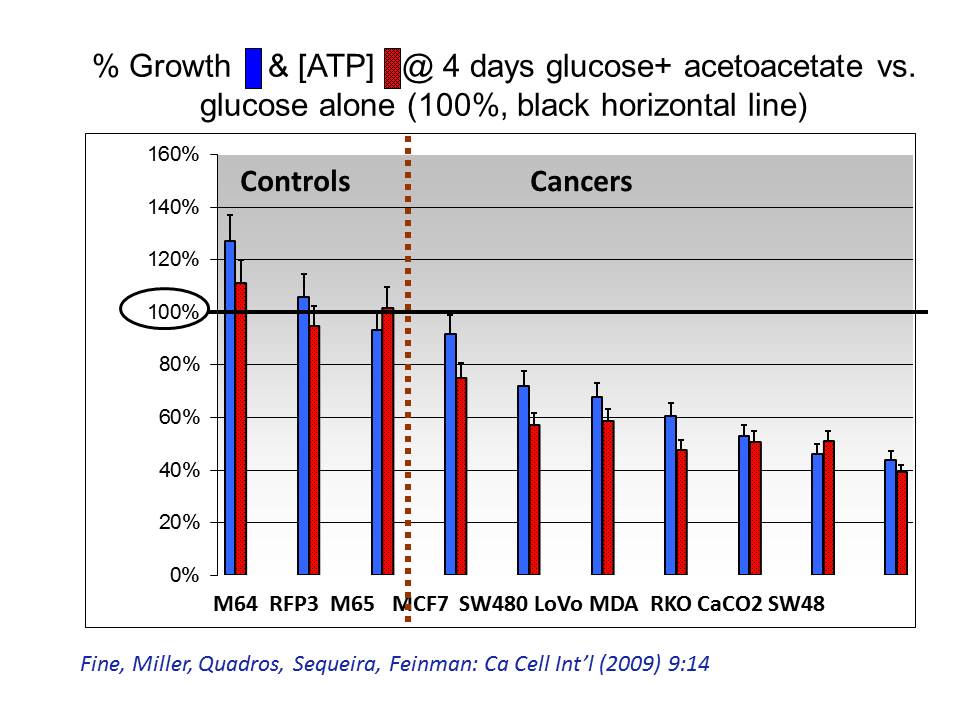
The graph shows growth (blue bars) and ATP (red) in cell lines after four days in ketone body supplemented growth medium. % marks on the vertical axis show how well the cells grew compared to the same cell lines grown in glucose medium alone. All 3 fibroblast lines (normal cells) grew and produced ATP at 100% (or more) of the rate with glucose alone. But all cancer lines had parallel reductions in growth and ATP when ketone bodies were added.
Project Backers
- 136Backers
- 268%Funded
- $10,001Total Donations
- $73.54Average Donation


