About This Project
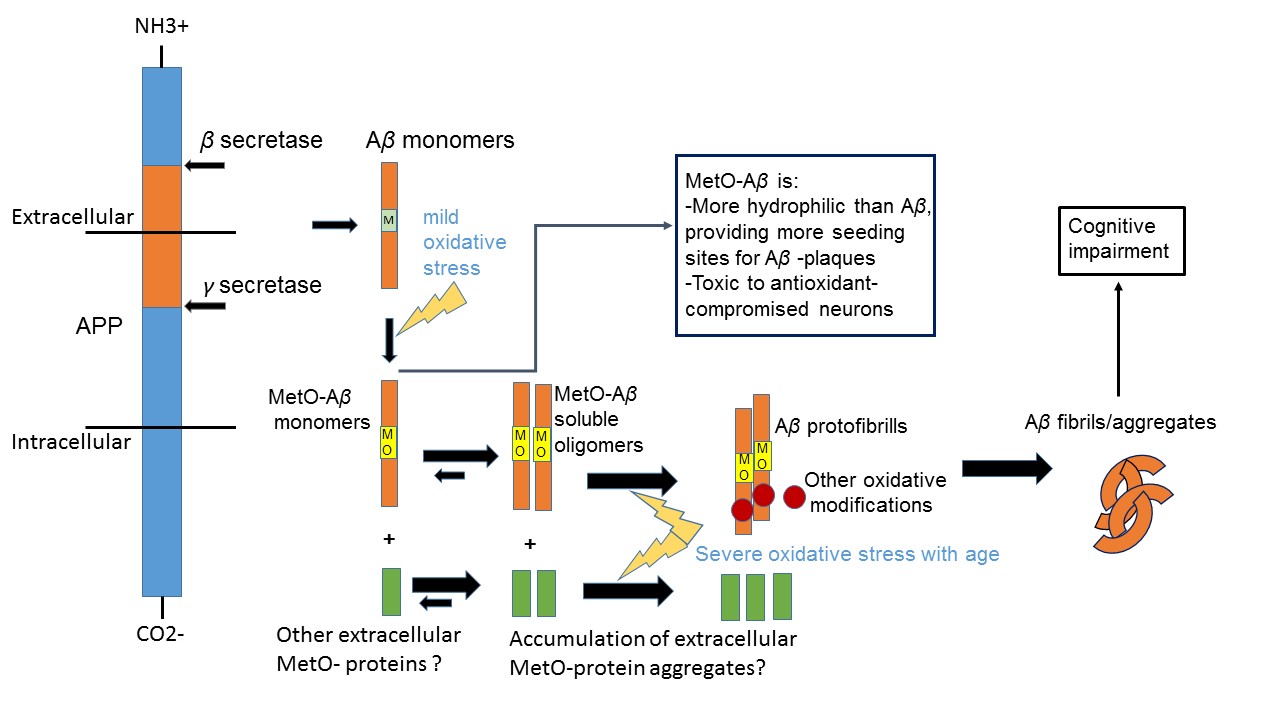
Oxidized forms of the peptide beta-amyloid (Aβ) are considered to be toxic to the brain, leading to the development of Alzheimer's disease (AD). Based on our preliminary data, we hypothesize that an immunization of an AD-model mice with a recombinant protein enriched with the oxidized amino acid methionine sulfoxide will cause the removal of toxic Aβ from brain. Consequently, this treatment is expected to inhibit the development of AD and alleviate cognitive impairment.
Ask the Scientists
Join The DiscussionWhat is the context of this research?
Accumulation of oxidized proteins with age may be one of the common causes of neurodegenerative diseases. Methionine sulfoxide (MetO) in proteins has been implicated in various protein malfunctions. Oxidation of the sole methionine (Met35) of Aβ may be one of the earliest events contributing to the toxicity of Aβ in-vivo. Previous attempts to remove Aβ from human AD-patients, by immunization against abnormal forms of Aβ, failed to show promising results. Thus, our alternative approach is to use (instead of Aβ) a surrogate antigen (MetO-rich protein). Previously, we have showed that inducing the production of anti-MetO antibodies in an animal model of AD significantly reduced hippocampal and cortical Aβ burden. Thus, reduction in dementia will be tested.
What is the significance of this project?
Oxidative stress is increased with an advanced age causing the accumulation of the toxic form of Aβ, Aβ-MetO. This form oxidized form of Aβ has a higher solubility in brain, providing better mobility and access to neurons throughout the hippocampal and cortical regions of the brain. Accordingly, clearance of Aβ-MetO from brain may prevent or reduce AD-associated cognitive decline. Current clinical trials immunotherapy treatments are based on periodic injections of antibodies to AD patients. So far, This approach showed low success rates. Even if this treatment becomes successful, it still potentially has the risk of being rejected by the host immune system with time (besides the need of monthly injections) .
What are the goals of the project?
Our hypothesis is auto-antibodies made against injected MetO-rich protein (antigen) could be used as a treatment for AD through elimination of brain MetO-Aβ (suggested to be an early-oxidized form of Aβ) and possibly other MetO-containing proteins that mediate Aβ toxicity. Our overreaching goal is to determine the ability of the antigen to alleviate memory-related phenotypes associated with AD. The APP/PS1 mice show deficiencies starting at 6 months of age. Accordingly, the antigen will be administrated at age of 3-4 months (3 injections every 2 weeks). Then, the cognitive functions of the control and experimental mouse groups will be monitored at 8, 10 and 12 months of age for their cognitive abilities (We will use Y maze spontaneous alternation, and Morris water maze tests).
Budget
The budget items will enable us to purchase a particular AD-mouse strain that overexpreses the amyloid precursor protein (APP) , in which it’s AD behavioral prototypes start to appear only at age of 6 months (APP/PS1 strain). This characteristic will enable the immunization of the mice at age of 4 months prior to the exhibition of the phenotypes that will be assessed by our researchers. We will divide the mice into two groups (control and experimental) of ten animals per group. The funds will also support the production of the recombinant antigen to be injected to the experimental group of mice. The budget does not include expenses for animal housing and care, and labor needed for performing the experiments. Any amount of money raised above the posted amount will be used for these budget items.
Endorsed by
 Project Timeline
Project Timeline
We expect to start and complete the immunization and most of the behavioral testing in the first year of the project. Completion of the behavior studies will be performed at the start of the second year, depending on the aquired data. We also plan on starting and completing of the post-mortem analyses for key markers of AD in the future. This part will be pushed forward depending on the funding status.
Feb 27, 2019
Project Launched
Apr 01, 2019
Producing the antigen for the immunotherapy
May 01, 2019
Ordering AD mice from Jackson's Lab
Jul 01, 2019
Starting the immunization
Oct 01, 2019
Completion of the immunization and starting the behavioral tests
Meet the Team
Team Bio
Dr. Jackob Moskovitz' team members: Dr. Adam Smith, he is an expert in behavioral characteristics of mammalian animals at the University of Kansas. He will assist with the behavioral studies and analyses of the AD-mice used in this project. Dr. Philip Gao, he is an expert in recombinant DNA technology and protein purification. He is the owner and director of the KanPro company at the University of Kansas. He will assist in producing the antigen used in this project.
Jackob Moskovitz
During my PhD studies at the Technion (Israel Institute of Technology), I have investigated the role of the ubiquitin system in protein post-transnational modification and degradation under the supervision of Dr. Hershko and Dr. Ciechanover (2004 Nobel laureates in Chemistry). Since then, I have been engaged in research of another less explored but yet important protein post-translational modification that is enhanced upon oxidative stress, namely: methionine sulfoxide (MetO). I have extensively studied the role of MetO in biological systems, including brain, and its reversal by the methionine sulfoxide reductase (Msr) system in various organisms during my postdoctoral training at Hoffman La-Roche (Nutley, NJ) and National Institutes of Health (under the supervision of the Dr. E. R. Stadtman, RIP). During my training, I have created several Msr type A knockout organisms starting with bacteria, yeast, and lastly of a mouse. All of these knockout strains exhibit higher sensitivity to oxidative stress and methionine oxidation. After appointed to a faculty position at the University of Kansas, I have developed a novel anti-MetO antibody (US Patent No. 8,409,824,B2) that has been successfully used by several researchers for the identification of MetO- proteins in various biological systems. My current research involves the use of MsrA knockout mouse as a model and the novel anti-MetO antibody to investigate the effect of MetO on protein function and cellular regulation. More specifically, the focus of my research is to elucidate the role of methionine oxidation and reduction in brain and the role of oxidative stress in neurological disorders and diseases. Publications and citations record:
https://scholar.google.com/cit...
Besides research, I enjoy painting on canvas, playing the piano and playing basketball in my free time.
Adam Smith
I have a broad background in neuroscience and pharmacology, with specific training and expertise in cellular and systems analysis of neuroendocrinological mechanisms of stress, learning and memory, and social behavior. In particular, my scientific interests encompass the role of signaling peptides on regulating the (1) formation and maintain of close relationships and lasting memories and (2) bi-directional relationship between stress and sociality. I have the experience and expertise to conduct behavioral assessments on animal behavior and cognition, including memory tasks, anxiety, depression, and social behavior. In addition, I have extensive experience with measuring protein concentrations and gene expression in a multitude of species from numerous biological samples and preparations. The long-term goal of this research is to deconstruct the neurobiology of sociality (including social memory, affiliation, and preference) and develop novel therapeutic agents for the treatment of social and emotional disorders such as generalized anxiety disorder, social anxiety disorder, and depression. In my collaboration with Dr. Jackob Moskovitz, we have developed bio-behavioral assessments of neurodegeneration and cognitive and behavioral decline associated with Alzheimer’s diseases (AD) within a AD mouse model.
Philip Gao
Additional Information
Project Backers
- 45Backers
- 32%Funded
- $2,552Total Donations
- $56.71Average Donation







